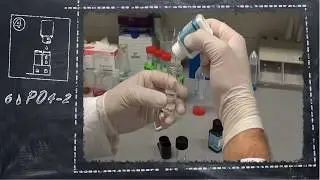
Nitrat / Nitrate
Online Tutorial materials in Biology for the education department at the LMU.
Lehrerbildung@LMU
Sodium nitrate is the most important, naturally present nitrate. The main deposit is in
the dry areas of northern Chile. Therefore, it is sometimes referred to as Chile saltpeter.
Additional small deposits are known in Egypt, Colombia and California. Due to
weathering of potassium rich soils, potassium nitrate mainly occurs in India, China
and Egypt. Formation of nitrates can be explained mainly due to the conversion of
proteins (e.g. from dead sea algae in the case of sodium nitrate) as a result from microbial nitrification. A special formation is calcium nitrate (e.g. saltpeter from masonry). From decomposing proteins, bacteria form in oxidizing processes first ammonia and then nitric acid. Nitric acid then forms together with the calcium carbonate within the masonry calcium nitrate. A group of plants that indicate nitrate in soil and, to some extent, can store nitrate very well are called nitrate plants (nitrophilous). Examples for nitrophilous plants are willowherbs, nettles, hogweeds and cow parsley, which occur in large populations in wet lands or tilled soil with high nitrate content.
To evaluate the self-purification capacity of a water body, it is important to test if there
are high amounts of ammonium and nitrite present as well. If these other parameters
are not high, the self-purification capacity to mineralize organic compounds is sufficient. This relation also holds true for the outflow of biological waste water treatment facilities. In drinking water, 50 mg/L nitrate are set as the limit by WHO, European Union and the German water authorities alike.
The newly developed tests are based on our proven VISOCOLOR ® system and were developed as high-quality VISOCOLOR ® SCHOOL test specifically for schools.
Director and creator of the film:
VIKTORIIA KAMSKA
Played by:
Immanuel Görens
Production:
Dr. Martina Bryce and Dr. Jennifer Flech