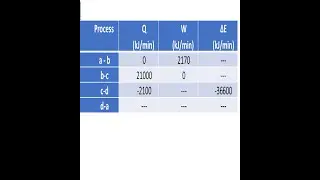
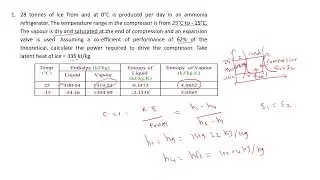
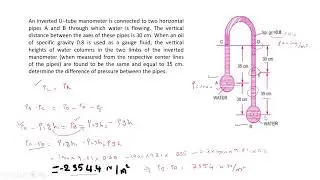
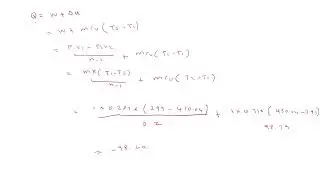
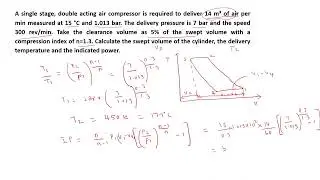
Gate Problem related to First Law of Thermodynamics
#Afirstlawofthermodynamics #internalenergy #workdone #heattransfer #surroundings
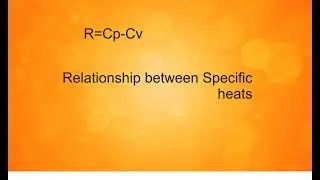
A gas contained in a cylinder is compressed, and the work required for compression is 5000 kJ. During the process, heat interaction of 2000 kJ causes the surroundings to be heated. Determine the change in internal energy of the gas during the process.