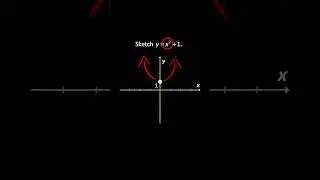
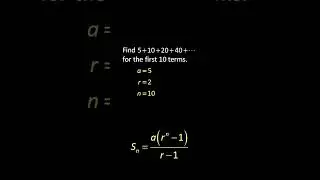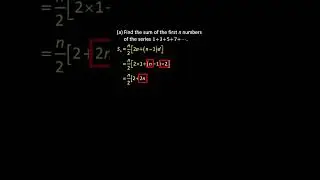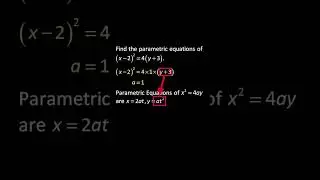💯 The Periodic Trends of Oxides Explained in Detail with Examples
📢 Receive Comprehensive Mathematics Practice Papers Weekly for FREE 😊
Click this link to get: ▶️▶️▶️ https://iitutor.com/email-list/ ◀️◀️◀️
General trends of oxides in the Periodic table
• As metallic character increases, the basic property of an oxide increases.
• Oxides of elements on the left-hand side of the Periodic table are basic. Moving to the left of the Periodic table, the basic property of an oxide increases.
• Oxides of elements on the right-hand side of the Periodic table are acidic.
• Some of the metallic oxides are amphoteric
• Some non-metallic oxides are not soluble in water and neutral
• Group VIII elements do not form oxides.




![mafia??? [gta in desc]](https://images.mixrolikus.cc/video/-YRyqGv_Bfs)