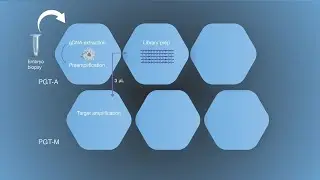
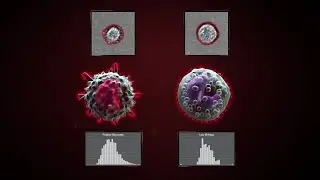
Digital PCR Measures Her-2 Copy Number in Breast Cancer Samples (Dr. Zoppoli at AACR 2013)
Learn more at: http://www.lifetechnologies.com/quant...
Dr. Gabriele Zoppoli of the University of Genova, Italy presents digital PCR data from breast cancer samples using the QuantStudio 3D Digital PCR System. This presentation took place Washington D.C. before the 2013 American Association for Cancer Research Conference. In his presentation, Dr. Zoppoli focuses on ERBB2 (her-2) amplification, which leads to a more aggressive phenotype. Because only her-2 amplified cancers are treated with and respond to treatment with monoclonal antibodies targeting the ERBB2 protein product (Her-2/neu), accurate her-2 amplification status assessment is of the utmost importance in breast cancer treatment decision-making. He describes the use of dPCR in a set of equivocal ERBB2 BC patients to assess copy number. Dr. Zoppoli also discusses digital PCR results compared to those obtained by qRT-PCR, IHC, and aCGH methods.
Transcript:
So what we do today, what we're talking today is about the use of digital PCR QuantStudio 3D system to measure HER2 in borderline amplified breast cancer samples. So a brief introduction about HER2, or bB2 as the official nomenclature goes. So as most of you know, HER2 is amplified in up to 15, 20% of breast cancer patients. This leads in turn to overexpression of HER2, and to an aberrant proliferation and a worse prognosis. Since there are targeted therapies that are mainly antibodies against HER2, and now there also new antibodies targeting the binding of HER2 to HER3, it is of the utmost importance to identify those patients that actually have HER2 amplification in order to make a proper decision for their treatment.
So here's just an example, by array CGH on a cell line that we used as a positive control. Array CGH shows a high level amplification of the HER bb2 locus. So as I was mentioning again, HER2 is focal in breast cancer. It is part of the HER family of membrane tyrosine kinases. So upon amplification, HER2 is overexpressed. Overexpression leads - goes to protein overexpression. And protein overexpression in itself is sufficient to drive breast cancer cell proliferation, because HER2 homo-dimerizes or hetero-dimerizes with other members of the family, and activates a plethora of downstream targets, which are in themselves, now targeted by targeted therapies. So this is a very important pathway. Examples are [PH] P-arthrikanese, [PH] M-Tor, [PH] B-Roff, and [PH] Kirase, which is upstream, and [PH] SOC.
So there is an issue in HER2 status determination, which is basically that you have to identify the amplification. So [PH] ASCO, and the college, and the College of American Pathologists identified level of tests to be performed in breast cancer to identify those patients. So basically, the first line is immunohistochemistry. And there are scores which go from zero to 3+, 3+ being highly positive and no doubt about it, treat it - the tumor, I mean, yeah. Zero is negative. 1+ is negative. 2+ is equivocal. It means no uniform, or weak complete, greater than 10%, but smaller than 30%; cells are stained for HER2 antibody. So what shall we do then? Proceed to a second level test, like florescence and cytohybridization. So this can be done by either single color FISH or dual color FISH. Single color FISH is basically, you take a big FISH probe, 100 kilo bases or something, like targeting HER2 locus. And you count the number of HER2 foci in each nucleus, and the number of nuclei ranging from a minimal of 20 to up to whatever you want, to be sure that there is actually an amplification or there is no amplification. Or, you can take two probes - one in the centromeric region, and one on the HER2 locus, and perform a ratio. So if the ratio is below a certain threshold, you have a negative sample; same goes for the absolute average count. If the average count is above 6, which was chosen as a cut-off, you have an amplified breast cancer. Again, you got equivocal results. In the clinical community, we don't treat these cancers with anti-HER2 drugs. So we're not still sure that it's beneficial, actually.





![КАК НАУЧИТЬСЯ ИГРАТЬ НА ЛЮБОМ ГЕРОЕ ВСЕГО ЗА 1 ИГРУ ? [ДОТА 2]](https://images.mixrolikus.cc/video/7eCG-8AEj5M)